
Who Is Making the COVID Vaccine?
As people across the United States consider receiving the Coronavirus vaccines, many are left with questions about the different vaccine brands. Because there are currently three authorized and recommended vaccines available to Americans, those seeking the shot may be unsure about who is making the COVID vaccine, which brand is safest, and if certain shots are more effective than others. Keep reading to learn more about the vaccine developers, so that you have all the information before you step into line at your local vaccination location.

Pfizer-BioNTech
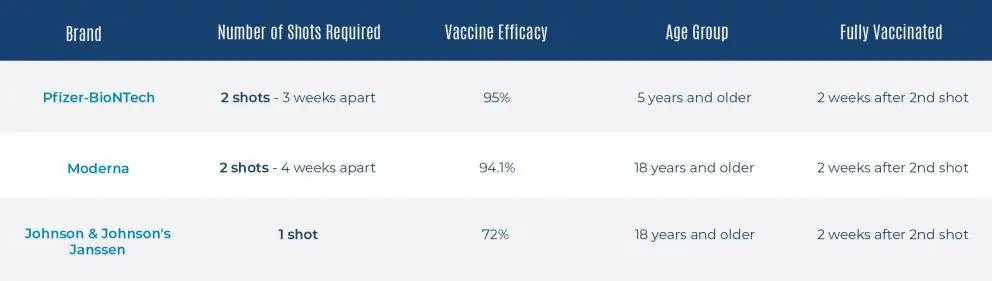
- Pfizer-BioNTech—referred to as simply Pfizer—was the first vaccine to be approved for the prevention of COVID-19 by the Food and Drug Administration (FDA). This vaccine is administered in two doses, taken roughly 3-4 weeks apart. Based on evidence from clinical trials, this vaccine was found to be 95% effective at preventing COVID-19 in people without evidence of previous infection.
Children’s Vaccination Availability
- Currently, the Pfizer vaccine is available to all children as young as five years old. Different dosages are given to children between the ages five and 11, 12 and 17, and those 18 and older.
COVID-19 Vaccine Booster Shots
- Experts recommend getting your Pfizer booster shot at least six months after completing your primary COVID-19 vaccination series. You are eligible for this booster shot if you meet one or more of the following guidelines:
- Completed COVID-19 vaccination series
- 65 years or older
- Age 18+ who live in long-term care settings
- Age 18+ who have underlying medical conditions
- Age 18+ who work or live in high-risk settings
Moderna
- The Moderna vaccine was granted emergency use authorization (EUA) on December 18, 2020. This shot was the second to be made available to the public and, like the Pfizer vaccine, is administered in two doses taken 28 days apart. Clinical trials have shown the Moderna vaccine to be 94.1% effective at preventing laboratory-confirmed COVID-19 illness in people who received the two doses and were not previously infected.
COVID-19 Vaccine Booster Shots
- Experts recommend getting your Moderna booster shot at least six months after completing your primary COVID-19 vaccination series. You are eligible for this booster shot if you meet one or more of the following guidelines:
- Completed COVID-19 vaccination series
- 65 years of age or older
- Age 18+ who live in long-term care settings
- Age 18+ who have underlying medical conditions
- Age 18+ who work or live in high-risk settings

Johnson & Johnson’s Janssen
- The third and final FDA and CDC-recommended vaccine is the Johnson & Johnson’s Janssen shot. Unlike its predecessors, this vaccine only requires one dose, so there is no need to plan for a second visit. When it comes to efficacy, the Johnson & Johnson vaccine was reported to have a 66.3% global efficacy rate and a 72% rate in the United States.

COVID-19 Vaccine Booster Shots
- Experts recommend getting your Johnson & Johnson Janssen booster shot at least two months after completing your primary COVID-19 vaccination series. You are eligible for this booster shot if you meet one or more of the following guidelines:
- Completed COVID-19 vaccination series
- Are 18 years of age or older
A Note on Booster Shots: The FDA has approved the mixing and matching of booster shots and COVID-19 vaccinations. The effectiveness of your booster shot you choose will not be compromised, regardless of the original vaccine you received.
Vaccine Side Effects
Side effects and their correlation severity varies from person to person and may also be unique to each vaccine, but there are some common side effects that people across the board have experienced.
Common side effects:
- Pain
- Redness
- Swelling
- Tiredness
- Headache
- Muscle pain
- Chills
- Nausea
These symptoms are completely normal and should subside within a few days; many can be managed with over-the-counter flu or pain medications. Most people report that they had more severe symptoms after their second dose of the Pfizer or Moderna vaccines. This immune response means that your immune system has developed antibodies against SARS COV-2.
Some rare symptoms, however, can be indicative of an allergic reaction or a more serious condition. If you have experienced a red, swollen, painful rash after receiving the Pfizer or Moderna vaccine, it is not recommended that you get the second dose. Another uncommon occurrence is the formation of Thrombosis (blood clots) after the Johnson & Johnson vaccine. It’s important to carefully monitor yourself and seek emergency medical attention if any of these severe symptoms occur.
FAQ
Are COVID-19 Vaccines Safe?
- All three of the aforementioned vaccines are approved by the FDA and therefore considered safe to be administered to the American public. The companies responsible for the research and development of the vaccine are also dedicated to creating safe vaccines. However, it’s important to monitor your body’s reaction after the vaccine; although uncommon, some people have experienced adverse allergic reactions to the shot. If you have known allergies to ingredients in other medicines or vaccinations, discuss with your doctor before getting the shot.
Should I get the COVID-19 vaccine if I had COVID-19?
- In short, yes you should get the vaccine even if you’ve already had COVID-19. However, it’s important to consult your doctor and discuss the severity of your coronavirus infection, as well as the onset of your symptoms. Your doctor may recommend waiting for your symptoms to fully subside before you take the vaccine.
Do Flu Vaccines Prevent COVID-19?
- The flu shot does not protect against contracting the coronavirus. Although the flu and COVID-19 do have similar symptoms, they are not the same virus.
Is the Moderna COVID-19 Vaccine Approved by the FDA?
- The Moderna vaccine was authorized for emergency use and distribution by the FDA on December 18, 2020. This status is currently still in effect but the CDC and FDA are continually monitoring each vaccine for safety and efficacy.
Educate Yourself and Get Vaccinated
It’s understandable to be nervous about receiving a relatively new vaccine. But to beat this virus, it’s essential that all vaccine candidates get the shot as soon as possible. If you have more questions about who is making the COVID vaccine or are experiencing concerning symptoms following the vaccine, do not hesitate to contact your doctor or visit an emergency center near you.
