
Monoclonal Antibody Treatment
ALL SYMPTOMATIC PATIENTS NEED TO BE SEEN BY A DOCTOR
What is Bebtelovimab Monoclonal Antibody Treatment?
Bebtelovimab is an investigational medicine used for the treatment of mild-to-moderate coronavirus disease 2019 (COVID-19) in adults and children (12 years of age and older weighing at least 88 pounds [40 kg]).
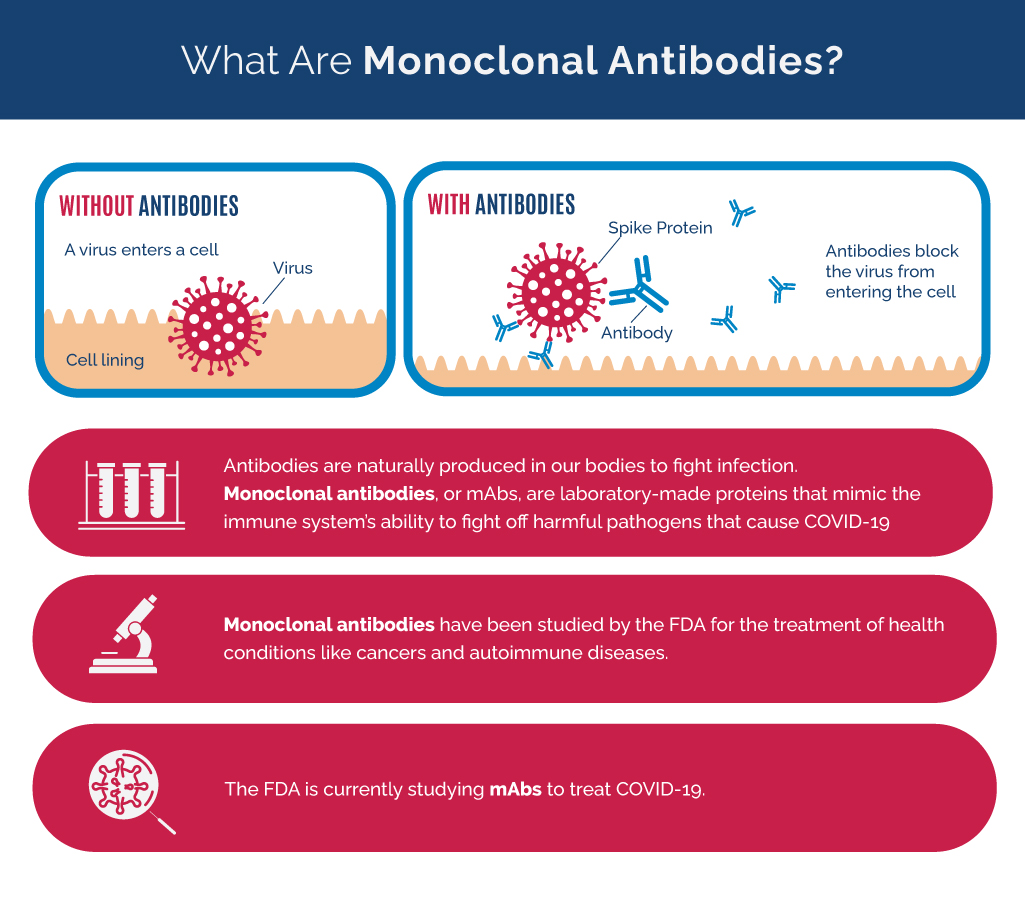
What Are Monoclonal Antibodies?
- Antibodies are naturally produced in our bodies to fight infection.
- Monoclonal antibodies, or mAbs, are laboratory-made proteins that mimic the immune system’s ability to fight off harmful pathogens at cause COVID-19.
- Monoclonal antibodies have been studied by the FDA for the treatment of health conditions like cancers and autoimmune diseases.
- The FDA is currently studying mAbs to treat COVID-19.
Antibody Cocktail Authorized Use
The United States FDA has made Bebtelovimab available under an emergency access mechanism called an Emergency Use Authorization (EUA). The EUA is supported by a Secretary of Health and Human Services (HHS) declaration that circumstances exist to justify the emergency use of drugs and biological products during the COVID-19 pandemic.
In issuing an EUA under the COVID-19 public health emergency, the FDA has determined, among other things, that based on the total amount of scientific evidence available, including data from adequate and well-controlled clinical trials, it is reasonable to believe that the product may be effective for diagnosing, treating, or preventing COVID-19, or a serious or life-threatening disease or condition caused by COVID-19; that the known and potential benefits of the product, when used to diagnose, treat, or prevent such disease or condition, outweigh the known and potential risks of such product; and that there are no adequate, approved, and available alternatives.
All of these criteria must be met to allow for the product to be used in the treatment of patients during the COVID-19 pandemic. The EUA for Bebtelovimab is in effect for the duration of the COVID-19 declaration justifying emergency use of Bebtelovimab unless terminated or revoked (after which Bebtelovimab may no longer be used under the EUA).
Who Can Receive the Bebtelovimab Treatment?
Bebtelovimab can be used to treat patients who:
- Have received positive results of direct SARS-CoV-2 viral testing
- Are at high risk for progression to severe COVID-19, including hospitalization or death
And
- For whom other COVID-19 treatment options approved or authorized by FDA are not available or clinically appropriate.
Possible Bebtelovimab Treatment Side Effects
Allergic reactions: Allergic reactions can happen during and after the injection of Bebtelovimab. Tell your healthcare provider right away if you or your child develop any of the following signs and symptoms of an allergic reaction: fever, difficulty breathing, low oxygen level in your blood, chills, tiredness, fast or slow heart rate, chest discomfort or pain, weakness, confusion, nausea, headache, shortness of breath, low or high blood pressure, wheezing; swelling of your lips, face, or throat; rash including hives, itching, muscle aches, dizziness, feeling faint, and sweating. These reactions may be severe or life-threatening. The side effects of receiving any medicine by vein may include brief pain, bleeding, bruising of the skin, soreness, swelling, and possible infection at the injection site.
These are not all of the possible side effects of Bebtelovimab. Not many people have received Bebtelovimab. Serious and unexpected side effects may happen. Not all of the risks are known at this time. It is possible that Bebtelovimab could interfere with your body’s own ability to fight off a future infection of SARS-CoV-2. Similarly, Bebtelovimab may reduce the body’s immune response to a vaccine for SARS-CoV-2. Talk to your healthcare provider if you have any questions.
